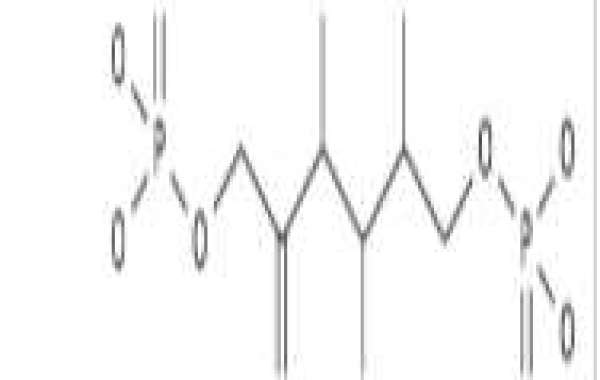
Excessive intake of sugar, including fructose (especially in sugar-sweetened beverages), may lead to insulin resistance, obesity, and elevated LDL cholesterol and triglycerides, leading to metabolic syndrome. In sugary foods and drinks, fructose may be preferred over sucrose and glucose because it has a lower effect on postprandial blood glucose levels, the European Food Safety Authority said, while also pointing to the potential for "high fructose intake to lead to metabolic complications". Adverse effects such as increased dyslipidemia, insulin resistance and visceral adiposity. [10][11] The Scientific Advisory Committee on Nutrition in the United Kingdom disputed the claim that fructose causes metabolic disorders in 2015, stating that “there is insufficient evidence that fructose consumed in normal British diets causes adverse health Effects irrelevant It exists as a component of total and free sugars.
Fructose is a six-carbon polyhydroxyketone. [14] Due to the stability of its hemiketal and internal hydrogen bonds, crystalline fructose adopts a cyclic six-membered structure called β-d-fructopyranose. In solution, fructose acts as tautomers β-d-fructopyranose, β-d-fructofuranose, α-d-fructofuranose, α-d-fructopyranose and keto-d-fructose (acyclic form) in an equilibrium mixture. [15]
The distribution of the d-fructose tautomer in solution is dependent on several variables such as solvent and temperature [16]. The distribution of d-fructopyranose and d-fructofuranose in water has been repeatedly determined to be approximately 70% fructopyranose and 22% fructofuranose.
Fructose undergoes Maillard reaction with amino acids, i.e. non-enzymatic browning. Because fructose exists more in open chain form than glucose, the initial phase of the Maillard reaction occurs faster than glucose. Thus, fructose has the potential to cause changes in the palatability of foods, as well as other nutritional effects such as excessive browning, reduced volume and tenderness, and the formation of mutagenic compounds during cake preparation.
The main reason fructose is used commercially in food and beverages is its relative sweetness, in addition to its low cost. It is the sweetest of all natural carbohydrates. The relative sweetness of fructose is reported to be 1.2-1.8 times that of sucrose. However, the six-membered ring form of fructose is sweeter.