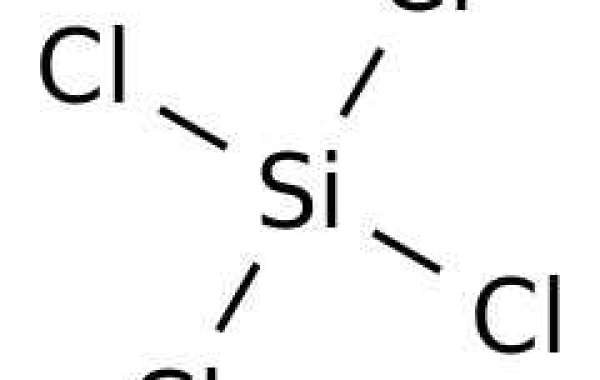Environmental Fate and Behavior
Silicon tetrachloride is a colorless, noninflammable, volatile liquid with a pungent, suffocating odor. It fumes in air and is corrosive to metals and tissues in the presence of moisture. In experiments at Argonne National Laboratory in which it was mixed with water and stirred under room conditions, about 35% of the theoretical yield of HCl evolved as a gas in the first minute. It also reacts very rapidly with alcohols, primary and secondary amines, ammonia, and other compounds containing active hydrogen atoms. Thermal decomposition or burning may produce dense white clouds of silicon oxide particles and hydrogen chloride.
Silicon tetrachloride is a by-product in the production of polysilicon, the key component of sunlight-capturing wafers in solar energy panels, and for each ton of polysilicon produced, at least four tons of silicon tetrachloride liquid waste are generated. Pollution by silicon tetrachloride has been reported in China, associated with the increased demand for photovoltaic cells that has been stimulated by subsidy programs.
Drawing Columns of Capillary Dimensions
Today, virtually all open tubular columns are prepared from fused silica and to a lesser extent borosilicate and soda-lime glasses. Fused silica is prepared by the oxidation of pure silicon tetrachloride in an oxygen plasma and is essentially pure silica, containing less than 1.00 ppm of metal impurities. The surface of fused silica glass is relatively inert, containing primarily silanol and siloxane groups. The presence of silanol groups is responsible for the residual acidic character of the glass. Because of its high melting point and ease of fracture, columns of capillary dimensions are drawn in a clean room facility at a rate of about 1 m s?1 from wide-bore silica tubes on a special apparatus, similar to those used for manufacturing optical fibers. The thin-walled tube must be protected immediately from moist air or dust particles that promote the growth of fissures or cracks. This is done by coating the outside of the capillary tube as it emerges from the softening furnace with a protective film of polyimide or aluminum. The drawn capillary column is inherently straight but sufficiently flexible to be coiled on a spool for collection purposes.
Nickel chloride is used for nickel plating cast zinc, as an agent in electrolytic refining of nickel, as a chemical intermediate for nickel catalysts and complex nickel salts, as an absorber of ammonia gas in industrial gas masks, as a catalyst in diarylamine and silicon tetrachloride production, as an agent in electrodeless plating of nickel, as an agent in tin–nickel alloy plating, and as a fungicide for control of rust and rustlike disease. However, workers exposed to different forms of nickel have an elevated risk of lung cancer. Besides, Ni and its compounds (particularly insoluble compounds of nickel) have been reported to be potent carcinogens and toxic agents in humans and experimental animals. Therefore, Ni compounds are considered to be an industrial/occupational health hazard.
Many inorganic chemicals are used as materials for optical applications. In particular, the optical fiber has been used for optical communications on a large scale and has had a major social influence in information communication. A necessary property of good optical glass materials is the transmission of information to distant places with little optical loss. Silica fibers (SiO2 fibers) are manufactured by lengthening silica glass rods produced from silica grains. The silica is made from ultrapure silicon tetrachloride (SiCl4), which is oxidized in the vapor phase by an oxyhydrogen flame. As the optical loss along fibers obtained by this method has already reached its theoretical limit, fluoride glasses are being used in the search for materials with lower levels of loss.
Finally, despite the myriad of paper studies, reliable property estimation is rarely successful for inorganic chemicals. The more difficult species to estimate accurately include the least soluble compounds, aluminates, borates, and some of the organometals. Improved solubility estimation for the difficult classes is likely as solution species are better defined. It is also likely that there is another variable needed to explain the activity of (and so estimate more accurately) the highly insoluble salts.