A recent article in The Des Moines Register newspaper has caused considerable controversy regarding nitrogen in Iowa streams and rivers. The article (High ammonia levels threaten D.M.’s water, April 6, 2008) featured information about “ammonia” levels in certain Iowa surface water systems during the recent winter time period.
The implications were that manure and fertilizer application to cropland, and subsequent snowmelt and runoff, had resulted in higher than normal “ammonia” levels in surface waters. In the article there was a comparison of the reported levels to an ammonia reading of 0.10 parts per million considered harmful to aquatic life. Unfortunately, measured surface water concentrations (and as reported in the article) are not ammonia-N. Instead they are ammonium-N plus ammonia-N. Therefore, a comparison of the reported values to a concentration of ammonia toxic to aquatic life is inaccurate.
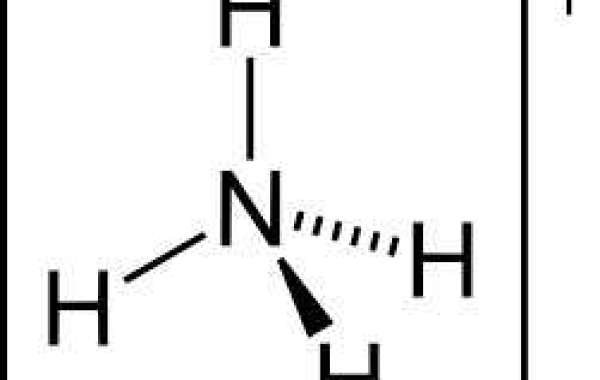
Ammonia is un-ionized, and has the formula NH3. Ammonium is ionized, and has the formula NH4+. The major factor that determines the proportion of ammonia or ammonium in water is water pH. The activity of ammonia also is influenced by temperature and ionic strength. This is important as the unionized NH3 is the form that can be toxic to aquatic organisms. The ionized NH4 is basically harmless to aquatic organisms.
When the pH is low, the reaction is driven to the right, and when the pH is high, the reaction is driven to the left. In general, at a temperature of around room temperature, at a pH less than 6.0, the proportion of ammonium-N plus ammonia-N as NH3 is very-very low and as NH4+ is very-very high. At a pH around 8.0, the proportion as NH3 is 10 percent or less, and at a pH slightly above 9.0, the proportion is about 50 percent. The activity of aqueous ammonia also is much lower at low temperatures and higher at warm temperatures. This means that at low temperatures and low pH the activity as NH3 is even lower, and as NH4+ is even higher. Therefore, sensitive aquatic organisms can tolerate a higher total “ammonium-N plus ammonia-N” at low temperatures than at high temperatures due to much less aqueous NH3 being present in the water.
Ammonia is a basic building block for ammonium nitrate fertilizer, which releases nitrogen, an essential nutrient for growing plants, including farm crops and lawns. About 90 percent of ammonia produced worldwide is used in fertilizer, to help sustain food production for billions of people around the world. The production of food crops naturally depletes soil nutrient supplies. In order to maintain healthy crops, farmers rely on fertilizer to keep their soils productive. Fertilizers also can also help increase levels of essential nutrients like zinc, selenium and boron in food crops.
The laboratory method used for analysis of water measures ammonium-N plus ammonia-N. It is very difficult to directly determine the activity of aqueous ammonia, so instead the surrogate of ammonium-N plus ammonia-N is used, and then tabled values of ammonium-N plus ammonia-N are used to determine if a measured concentration will provide ammonia at a level that is detrimental to aquatic organisms, for acute and chronic conditions. These tabled values are a surrogate since the measured concentration is a total of the ammonium-N plus ammonia-N, and the concentrations in the tables for chronic or acute levels are set to reflect back to likely concentrations of ammonia-N for specific water pH and temperature.
The acute and chronic criteria for “ammonia” have been established for Iowa streams designated for aquatic life uses (Chapter 61, Iowa Administrative Code; tables 3a, 3b and 3c). One has to carefully use the tables as the listed concentrations are for ammonium-N plus ammonia-N, not ammonia-N (the header to the tables says “ammonia”). As expected, chronic criteria (ammonium-N plus ammonia-N concentration) are higher for low pH and low temperature water (ex. pH 6.5 at 0 degrees C is 6.67 mg N/l, early life stages present) and lower for high pH and high temperature water (ex. pH 8.0 and 26 degrees C is 1.16 mg N/l). Similarly, acute criteria are higher for low pH water (ex. at a pH of 6.5 the criteria for class B (WW1-3) and B(LW) is 48.8 mg N/l) and lower for high pH water (ex. at a pH of 8.0 is 8.4 mg N/l). The early February 2008 ambient monitoring levels from the Iowa Department of Natural Resources Storet database for the rivers identified in The Des Moines Register article ranged from 0.13 to 1.00 mg N/l (ammonium-N plus ammonia-N). At the water pH and temperature during that time (7.6 to 8.1 pH and 0 to 1.0 degrees C), the monitored values are well below both acute and chronic criteria for those conditions (acute criteria 17.0 to 6.95 mg N/l and chronic 3.98 to 2.10 mg/l). Measured ammonium-N plus ammonia-N tends to be variable during the winter months, but the variation and levels this year are not higher than recent history.