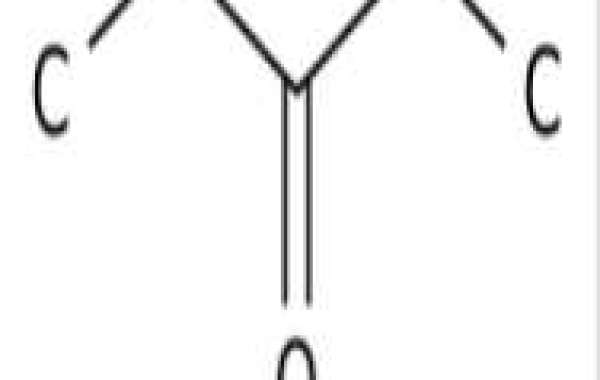
3-Pentanone also known as diethylketone or ethylpropionyl, is a member of the ketone family. A ketone is an organic compound in which a carbonyl group is bonded to two carbon atoms R2C=O (neither R can be a hydrogen atom). Ketones with one or more α-hydrogen atoms undergo keto-enol tautomerization, the tautomer being an enol. Therefore, 3-pentanone is considered to be an oxygenated hydrocarbon lipid molecule. 3-Pentanone is soluble (in water) and is a very weakly acidic compound (based on its pKa). 3-Pentanone is an acetone and bland compound found in many foods such as strawberry guava, Ceylon cinnamon, beech nuts, and cabbage, making 3-pentanone a potential biomarker for the consumption of these foods.
3-Pentanone is mainly used as a solvent for paints and a precursor of vitamin E. Used as a reagent for the synthesis of 2-cyano-3,3-diethylacrylate by Knoevenagel condensation. It also showed anticonvulsant effects in several types of mouse seizure models. It is used as an intermediate in the manufacture of medicines.
A single-atom Ru catalyst for the selective synthesis of 3-pentanone by hydroformylation of ethylene.
3-Pentanone was synthesized by heterogeneous ethylene hydroformylation using Ru single-atom (Ru SA) catalysts supported on activated carbon with a selectivity of 83.3%, while the ethane selectivity of Ru nanoparticles (Ru NPs) was 52.1% %. Atomically dispersed Ru species with an oxidation state (Ruδ+) and a Ru-C4O coordination structure were identified as active sites for efficient C–C coupling to 3-pentanone, while metallic Ru nanoparticles exhibited a strong response to the hydrogenation of ethylene to ethane High activity. Density functional theory (DFT) calculations revealed that the energy barrier for the direct coupling of C2H5CO* to C2H5* to form 3-pentanone on Ru SA is much lower than that of Ru NPs. Therefore, the formation of 3-pentanone on Ru SA is more favorable than propanal, which is produced by the coupling of C2H5CO* and H*. This strategy may provide a potential green route for the atom-economical one-pot synthesis of 3-pentanone.