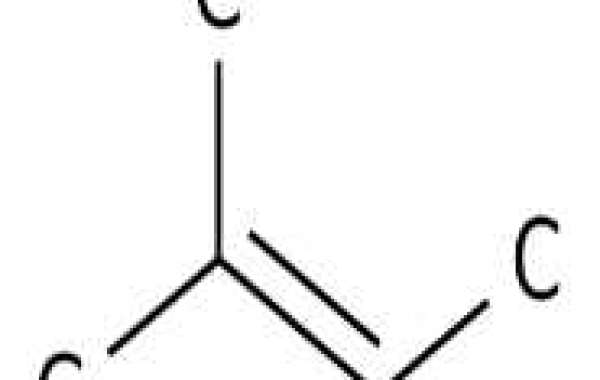
2-Methyl-2-butene may react violently with strong oxidizing agents. May react exothermically with reducing agents liberating gaseous hydrogen. Exothermic polymerization reactions may occur in the presence of various catalysts (e.g. acids) or initiators.
Kinetic modeling is especially valuable when new experiments and kinetics are combined
Modeling can address fundamental processes that have a significant impact on practical combustion problems. The present work involves the oxidation of 2-methyl-2-butene (2M2B), a small unsaturated
Branched-chain hydrocarbon fuels, to the best of our knowledge, although they are used as intermediates in
Combustion of many larger hydrocarbon fuels. New experimental results for 2M2B shock tube ignition and jet stirred reactor (JSR) oxidation are presented below, along with detailed chemical kinetics
Reaction mechanisms were developed to describe the reactions involved in the two sets of experiments.
2-methyl-2-butene is a natural product found in Tuber borchii for which data are available.
Many (but not all) of the experimental results obtained in JSR are symbolized in Figures 4-6, and all experimental measurements are summarized in the Supporting Information
in tabular form. These figures show that 2-methyl-2-butene starts to react at about 700 K, with little reactivity observed at lower temperatures.
Overall, there are few sources of experimental data to aid in the development and testing of kinetic mechanisms for C5 and larger olefinic fuels. It is also surprising that no past experiments or kinetic modeling papers have provided any intermediate species measurements for branched unsaturated hydrocarbon fuels larger than isobutene. Current Experiments and Kinetics