Phosphoric acid is a major chemical product with many important uses, especially in fertilizer production. Most acids are produced from phosphate rocks. Crude H3PO4 from the challenge stage was filtered and concentrated in an evaporation unit [33]. Steel used in the phosphoric acid industry needs to be protected from corrosion. There appears to be little research on the inhibition of steel in H3PO4 solutions [34-39]. Fuda et al. [34] investigated some quaternary ammonium compounds as corrosion inhibitors for mild steel in 50% H3PO4. It was found that the corrosion mechanism of mild steel in H3PO4 does not change with the increase of acid concentration.
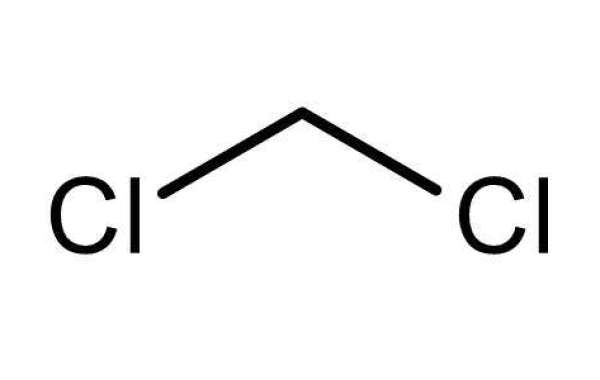
In this study, H3PO4 was used instead of HCl and H2SO4 because H3PO4 is an inorganic acid that is very useful in industry and medicine. Most commonly, it is used to remove dust from metal surfaces. It can also be used as a rust converter by placing it in direct contact with rusted iron, steel tools and other rusted surfaces. It converts reddish-brown iron oxide to black iron phosphate. When this iron phosphate is wiped off, the metal surface is refreshed.benzyltriphenylphosphonium chloride
Therefore, in the present study, the inhibitory effect of BTPPC on the corrosion of mild steel in 0.3 M H3PO4 solution was investigated by means of potentiodynamic polarization, potentiostatic polarization, electrochemical impedance spectroscopy, temperature kinetic studies, and scanning electron microscopy.
The working electrode (WE) for potentiodynamic studies was cut from a mild steel rod and welded at one end with an insulated copper wire, which was then embedded in chemical epoxy (ARALDITE), leaving 1 cm2 exposed surface area for for learning. The counter electrode was platinum and the reference electrode was a saturated calomel electrode (SCE) coupled to a Lukin capillary. The potential of the metal electrode is measured with the help of a galvanostat relative to a reference electrode. Steady-state potential is reached within 4-5 hours. The electrode system used for potentiostatic polarization studies and electrochemical impedance spectroscopy is the same as that used for potentiodynamic polarization studies. Potentiodynamic and potentiostatic polarization measurements were performed using an electrochemical analyzer CHI 6021B under gas-filled conditions. Potentiodynamic anodic and cathodic polarization curves were acquired at a scan rate of 0.001 Vs-1 over a potential range of -1.2 V to 0.2 V relative to the corrosion potential (Ecorr). Potentiostatic polarization curves were acquired at a scan rate of 0.01 Vs over the potential range from open circuit potential (OCP) to 2 V. Electrochemical impedance spectroscopy was performed using an electrochemical analyzer CHI760C under ventilation conditions. Impedance spectra were recorded at Ecorr in the frequency range 10000 Hz to 1 Hz. The AC voltage amplitude is 0.005 V.
Properly ground and polished mild steel samples (1 cm × 1 cm × 1 cm) were used for SEM. After soaking in a 0.3 M H3PO4 solution of 0.3 M H3PO4, 1×10-3 M BTPPC and 1×10-7 M BTPPC inhibitor for 24 hours at room temperature, the samples were removed from the solution and dried in a desiccator for 24 hours, and then These samples were used for SEM. SEM measurements were performed using a LEO 435 VP in high vacuum mode, equipped with digital imaging and a 35mm photography system. SEM images were acquired by applying an operating voltage of (15-30) kV.
Szukaj
Popularne posty
-
 Popeye’s Meets Chick-fil-A’s Fried Chicken Sandwich – Available Any Day, and All the Time
Wg fastfoodz
Popeye’s Meets Chick-fil-A’s Fried Chicken Sandwich – Available Any Day, and All the Time
Wg fastfoodz -
 Ebook SW OWED STAR аё аёІаё„ 13 аё•аёаё™аё—аёµа№€ 51 - Google Drive Epub Full Zip Download
Wg ncusexungai
Ebook SW OWED STAR аё аёІаё„ 13 аё•аёаё™аё—аёµа№€ 51 - Google Drive Epub Full Zip Download
Wg ncusexungai -
 Переломите стереотипы: Купить диплом и доказать свою компетентность
Wg worksale
Переломите стереотипы: Купить диплом и доказать свою компетентность
Wg worksale -
 Закажите необходимый диплом или аттестат на оригинальном бланке
Wg worksale
Закажите необходимый диплом или аттестат на оригинальном бланке
Wg worksale -
 온라인 카지노 검토 - 중요성 알기
Wg gulamali
온라인 카지노 검토 - 중요성 알기
Wg gulamali