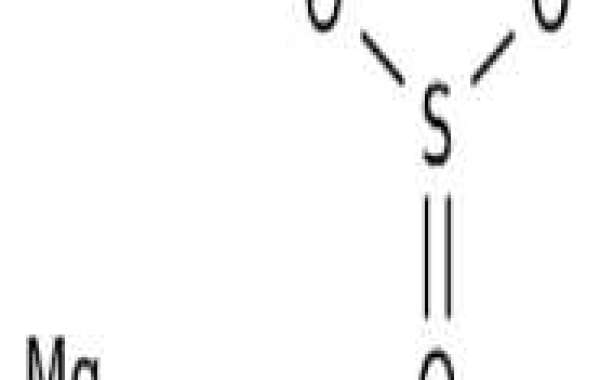
The oxidation of magnesium sulfite is of great significance for the recovery of by-products in magnesium desulfurization. The oxidation rate of magnesium sulfite prepared by vacuum evaporation was studied in the bubbler tank in the presence of transition metal catalysts, and it was shown that cobalt was the most effective. The general reaction orders of cobalt, magnesium sulfite and oxygen are 0.44, 0 and 0.46, respectively, and the apparent activity energy is 17.43 KJ·mol. The catalytic performance of cobalt compared with other metals was also analyzed using ionic potential theory. Combined with a three-phase reaction model, we deduce that the general oxidation rate of magnesium sulfite is controlled by oxygen mass transfer. In addition, intrinsic kinetics are predicted, showing reaction orders of 1.0 and 0 for cobalt and oxygen, respectively. The research results are helpful for the recovery and utilization of magnesium sulfite in magnesium oxide desulfurization.
Mechanism and kinetics of multi-walled carbon nanotube-catalyzed oxidation of magnesium sulfite.
Oxidation of magnesium sulfite is crucial for recovery of by-products in magnesium oxide desulfurization. Using an impregnation method, cobalt-supported multi-walled carbon nanotube catalysts were prepared to promote the oxidation rate of magnesium sulfite (MgSO3). The performance of impregnated catalysts with different concentrations of Co(NO3)2 was compared, and the results showed that the optimum impregnated concentration was 30%. Catalysts prepared with different concentrations of Co(NO3)2 were characterized by Brunauer-Emmett-Teller analysis, transmission electron microscopy, scanning electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. The kinetics of the catalytic oxidation of magnesium sulfite were studied in a bubbler tank, showing that the general reaction orders for carbon nanotubes, oxygen and magnesium sulfite were 0.22, 0.45 and 0.01, respectively. The apparent activation energy is 23.43 kJ mol−1. The three-phase reaction model suggests that the internal diffusion of oxygen may be the rate-determining step in the oxidation of magnesium sulfite. The research results can provide a reference for the design of multi-walled carbon nanotube catalysts for the oxidation of sulfite.