Iron(III) nitrate is the nitrate of iron. It is used as a catalyst in chemical synthesis and is also used by jewelers and metalsmiths to etch silver and silver alloys. Nitrite is a toxic compound known to cause methemoglobinemia.
Iron(III) nitrate or iron nitrate is the name for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. The most common is the nonahydrate Fe(NO3)3.(H2O)9. Hydrates are light-colored water-soluble paramagnetic salts.
Iron(III) nitrate is easy to deliquescence, and usually exists in the form of nonahydrate Fe(NO3)3·9H2O, forming colorless to lavender crystals. This compound is the trinitrate salt of the aqua complex [Fe(H2O)6]3+. [4] Other hydrates Fe(NO3)3 xH2O, including:
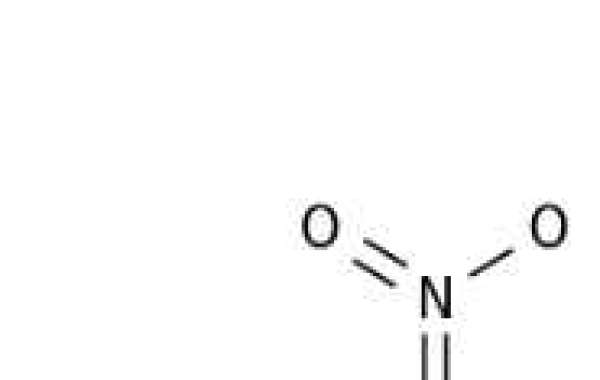
Fe(NO3) 3 dissolves in water to form an acidic solution. What is a chemical equation?
When dissolved in water, Fe(NO 3 ) 3 first ionizes into Fe +3 and NO-3 ions.
The Fe +3 ion then reacts with water to form the hexahydrate ion (III) ion Fe(H 2 O) +36 ;
Fe +3 + 6H 2 O → Fe(H 2 O) +36
On the other hand, the counteranion remains almost completely in the bulk solution; and the concentration increases with each cycle. A clever way to overcome it is to take advantage of its cumulative nature: it uses nitrate as a counter anion, i.e. iron nitrate (Fe(NO3)3) as a coagulant. In this way, the remaining nitrate ions after each harvest and medium reuse cycle can be used as a nitrogen source for the next round of cultivation. In this study, this hypothesis was tested and proven.
In this study, Chlorella sorokiniana was successfully cultured in circulating medium with nitrogen supplied directly by the coagulant Fe(NO3)3.