Succinimide is also used to form covalent bonds between proteins or peptides and plastics, which can be used in a variety of detection techniques.
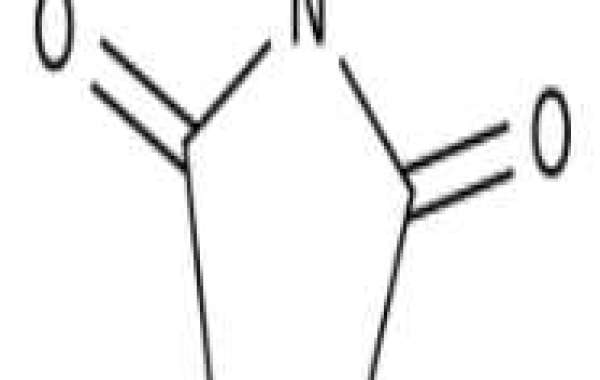
Succinimide is a dicarboximide which is a pyrrolidine substituted with oxo groups at the 2 and 5 positions. It is pyrrolidone and dicarboximide.
Succinimide is a dicarboximide which is a pyrrolidine substituted with oxo groups at the 2 and 5 positions. It is pyrrolidone and dicarboximide.
Succinimides and their derivatives are known to possess various therapeutic activities (Figure 7) [102-105] and are important building blocks in organic synthesis [106]. In 2016, Li/Ge reported a copper-mediated C–H activation strategy for the construction of a succinimide derivative 65 involving carbonylation of an aliphatic amide 1 (Scheme 37) [107]. Here, nitromethane acts as a carbonyl source. This reaction assisted dehydrocoupling of the C(sp3)-H of the amide with nitromethane via 8-aminoquinoline-assisted dehydrogenation followed by a Nef-type reaction to form the succinimide product 65. A related carbonylation reaction of aromatic amides to provide phthalimide derivatives also proved feasible under copper catalysis.
The conversion of 2-pyrrolidone to succinimide using oxygen-mediated photolytic radiation has been known since the 1980s. Gramain and colleagues studied the photolytic conversion of unsubstituted and substituted pyrrolidones to the corresponding succinimides. 43 When 2-pyrrolidone (1) was photolyzed in the presence of benzophenone in tert-butanol and molecular oxygen, succinimide (2) yielded at 60%. Similarly, N-methyl-2-pyrrolidone (3) was converted to N-methylsuccinimide (4) in tert-butanol in 60% yield (Scheme 2.17). The mechanism of oxidative transformation is hypothesized to involve free radical formation.
Containing succinimide and phthaloyl Trinaphthyl-Sb(V) compounds with imine ligands (see equation (20), Section 2.17.2.1) 2003JFC(122)165.