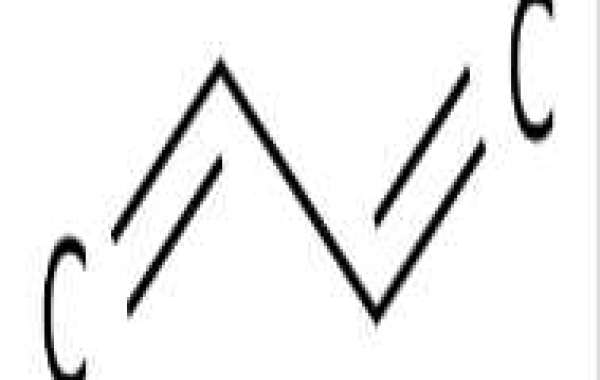
1,3-Butadiene (/ˌbjuːtəˈdaɪiːn/)[8] is an organic compound with the molecular formula CH2=CH-CH=CH2. It is a colorless gas that condenses easily into a liquid. It is industrially important as a precursor of synthetic rubber. The molecule can be seen as a combination of two vinyl groups. It is the simplest conjugated diene.
Although butadiene breaks down rapidly in the atmosphere, it is still present in ambient air in cities and suburbs due to continuous emissions from motor vehicles. [9]
The name butadiene can also refer to the isomer 1,2-butadiene, which is a cumulative diene with the structure H2C=C=CH−CH3. This propadiene has no industrial significance.
In 1863, French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol [10]. The hydrocarbon was identified as butadiene in 1886 after Henry Edward Armstrong isolated it from the pyrolysis products of petroleum. [11] In 1910, Russian chemist Sergey Lebedev polymerized butadiene to obtain a material with rubber-like properties. However, this polymer was found to be too soft to replace natural rubber in many applications, notably car tires.
The butadiene industry originated in the pre-World War II era. Many belligerent nations realized that in the event of war they might be cut off from the rubber plantations controlled by the British Empire and sought to reduce their dependence on natural rubber. [12] In 1929, Eduard Tschunker and Walter Bock, working at IG Farben, Germany, produced a copolymer of styrene and butadiene that could be used in automobile tires. Global production soon followed, with butadiene produced from grain alcohol in the Soviet Union and the United States, and from coal-to-acetylene in Germany.
Butadiene is a by-product of the steam cracking process that produces ethylene and other olefins in the United States, Western Europe and Japan. When mixed with steam and briefly heated to very high temperatures (often in excess of 900 °C), aliphatic hydrocarbons release hydrogen gas, forming a complex mixture of unsaturated hydrocarbons including butadiene. The yield of butadiene depends on the hydrocarbons used as feed. Light feeds, such as ethane, produce primarily ethylene when cracked, but heavier feeds favor the formation of heavier olefins, butadiene, and aromatics.