Ethyl acetate boiling point is a widely used solvent, especially for paints, varnishes, lacquers, cleaning mixtures, and perfumes. Like last week's MOTW, dichloromethane, it is used as a solvent for decaffeinating coffee beans. In the lab, ethyl acetate boiling point is a common solvent for column and thin-layer chromatography.
Ethyl acetate boiling point (EtOAc) is an effective alternate solvent for diethyl ether, employed for the concentration of eggs, larvae, and cysts in fecal specimens during the Formalin-ether sedimentation technique.[1] Acetaldehyde is reaction intermediate formed during the oxidative combustion of ethanol and ethyl acetate boiling point in the presence of copper oxide embedded on Ce-doped titania surface.
Ethyl acetate boiling point appears to be a satisfactory subsitute solvent for diethyl ether in the Formalin-ether sedimentation technique. In comparative studies, concentration of organisms with ethyl acetate boiling point was equal to or greater than that with diethyl ether. No distortion or alteration
Ethyl acetate boiling point is one of the simplest carboxylate esters. (Former Molecule of the Week methyl formate is the simplest.) The colorless liquid has a sweet, fruity odor that most people find pleasant.
As you might expect, ethyl acetate boiling point was first synthesized from ethanol and acetic acid. The reaction was the classic acid-catalyzed Fischer esterification, which dates back to 1895. This is still the most widely used commercial synthesis. An alternative method is the Tishchenko reaction in which acetaldehyde disproportionates in the presence of base to the alcohol and the acid that then esterify in situ.
Ethyl acetate boiling point is a widely used solvent, especially for paints, varnishes, lacquers, cleaning mixtures, and perfumes. Like last week’s MOTW, dichloromethane, it is used as a solvent for decaffeinating coffee beans. In the lab, ethyl acetate boiling point is a common solvent for column and thin-layer chromatography.
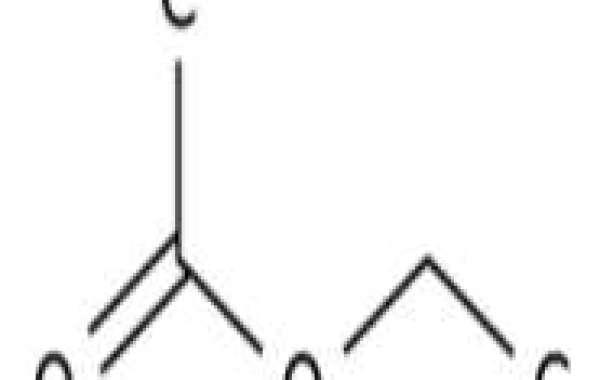
Ethyl acetate (systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate boiling point is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.
Ethyl acetate boiling point was first synthesized by the Count de Lauraguais in 1759 by distilling a mixture of ethanol and acetic acid.[6]
In 2004, an estimated 1.3 million tonnes were produced worldwide.[5][7] The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tonnes. The global ethyl acetate boiling point market was valued at $3.3 billion in 2018.[8]
Ethyl acetate is synthesized in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in about 65% yield at room temperature:
CH3CO2H + CH3CH2OH → CH3CO2CH2CH3 + H2O
The reaction can be accelerated by acid catalysis and the equilibrium can be shifted to the right by removal of water.